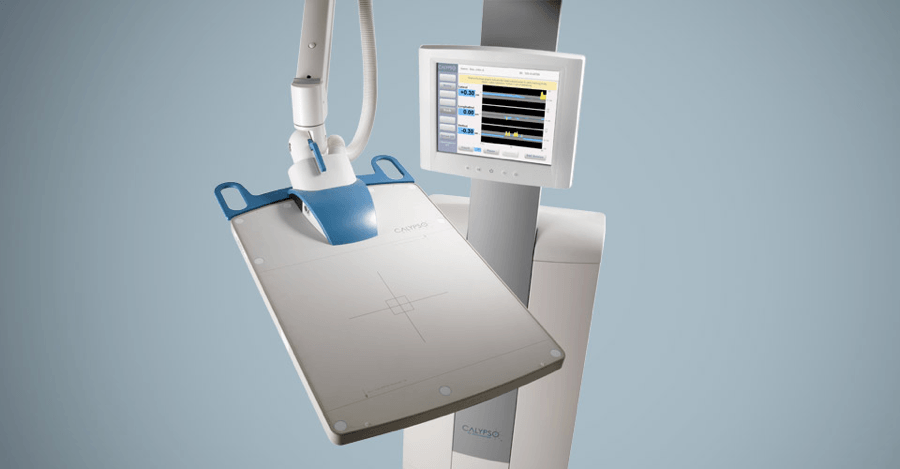
In 2001, I joined Calypso Medical as employee number 18. Our goal was to create a remarkable medical device that could track the location of the prostate to a millimeter of accuracy during prostate cancer treatments.
This level of accuracy is important because the prostate has a tendency to move unpredictably during normal bodily functions, like coughing, going to the bathroom, or passing gas. This makes it difficult to direct the radiation to the correct spot. Healthy tissue may accidentally receive the radiation, which can lead to increased side effects.
We called it GPS for the body. Rather than satellites whizzing around the earth to pinpoint your phone’s location, a sensor array the size of a pizza box hovers directly over the patient’s abdomen. This sensor communicates with three transponders, about the size of a grain of rice, that had been implanted in the prostate in an earlier procedure.
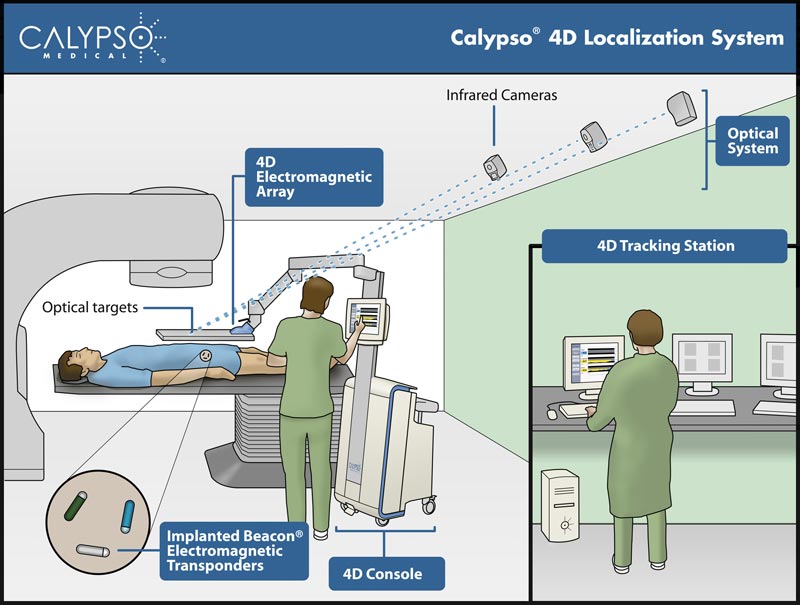
During treatment, the radiation technologist (RT) monitors the location of these transponders. If the prostate moves outside of the radiation beam, the RT is immediately alerted and can reposition the beam so that it is once again focused squarely on the tumor. If you know where the device is, you know where to target the radiation.
For this to work, we needed another system that could determine the location of the sensor array. Figuring out the best way to solve that problem was my job.
Walk a mile in your users’ shoes.
As is typical in small companies, everyone wore multiple hats. If I wanted to understand what was happening during treatment and how it would constrain my system, I would need to figure that out myself.
Luckily, a local hospital was very helpful and let me hang out with the RTs as they did their job. I watched how they aligned the patients and moved about the room and spoke with the medical physicists about how they calibrated and aligned the equipment. I needed to design my system to work with what was already happening. Ideally, it would be invisible to the RTs and patient.
Build prototypes to simulate products in real-world settings.
After exploring several options, I settled on a ceiling mounted camera system that would see the array and could figure out its location. I used three cameras, even though two would be enough, so that the RTs could move about the room and not worry if they were blocking one of the cameras.
I developed simulations and was confident the system would work. But a prototype is much more convincing and can test errors in your assumptions that a simulation might miss.
I built the prototype with commercial-off-the-shelf tripods and cameras and software that I wrote. In testing we showed the concept worked even if you blocked a camera or the targets.
I then installed my prototype in an unused treatment space at the hospital, and we were able to simulate realistic usage. This work convinced the company leadership that I was on the right track.
Choose your partners carefully.
Once everyone agreed that my concept would work, I was directed to select a partner to implement my concept in a way that would pass muster with the FDA.
The perfect partner would have certain features:
- An existing solution that could be leveraged for our needs
- Their team had the desire and ability to customize their solution
- Their solution had been through the FDA regulatory approval process
- Geographically close to our office in Seattle
- Reasonable business terms
- A team that would be easy to work with over the long-term
- A company that was stable enough that we didn’t have to worry about them going out of business
Not surprisingly, no such company existed.
One company had an FDA-approved camera-based solution, but the solution didn’t have the resolution we needed and wouldn’t work if someone walked in front of a camera. Any solution they created would have to be built from scratch.
Another company was a spin-off of a university in Munich, Germany. Their solution was technically solid, but they were a startup with no other customers and definitely not geographically desirable.
A third company had a technically solid solution and several customers in the movie business. They were a leading company for motion capture and had worked on movies like “The Hobbit”. Their location in California was not ideal, but at least they were in the same time zone and a single flight away.
The only missing element was that their device hadn’t been through an FDA approval process. We worked with a regulatory consultant and the company to develop an approach that worked for everyone. It’s been over 15 years, and this partner is still providing the camera system for the Calypso tracking system.
Anticipate and prevent product failures using failure mode and effects analysis.
When designing a medical device, it’s critical that it works as it’s supposed to. The alternative can be the death of the patient. One of the tools that we used to accomplish this was failure mode and effects analysis (FMEA), a structured way to analyze how a product might fail and what you can do to prevent it. In this context, failure means the product doesn’t deliver the required performance, not that it stops working.
For instance, if your requirement is accuracy no worse than 1.0 mm and a condition results in a location error of 1.1 mm, that’s a failure.
FMEA typically starts with a brainstorming session where you identify ways that failure might happen. Our failure modes included:
- Changes in the room temperature causing the camera mounts to move, pushing the system out of calibration.
- The radiation environment in the treatment vault (both gamma rays and neutrons) causes cameras to fail.
- Partial obscuration of the targets on the array, leading to an inaccurate location solution that doesn’t trigger an error condition.
For every failure mode we stated a severity (how bad would it be if this happened) and an occurrence (how likely would it be to happen). For example, a failure mode that shuts down the system (like a dead camera) would be high, but not the worst. The most severe failure mode is one that could lead to accidentally targeting radiation to the bowel or bladder, resulting in serious side effects.
An interesting failure mode that we discovered was exposure to neutrons, sub-atomic particles with no charge. The process of creating the beam of radiation used to kill the cancer cells also created a flood of free neutrons that might damage our electronics. I flew our cameras to one of the only two neutron test sites in the U.S. and exposed our camera to 10 years’ worth of neutrons in a few hours.
From that test, we learned that one component was sensitive to neutrons and needed to be replaced.
If we hadn’t done the FMEA work, our cameras would have started failing in the field. Until we figured out the pattern of failures, the cameras would have just been replaced. Once the root caused was determined, we would have needed to replace the part and recertify the cameras, delaying new installations. This would have hurt our reputation, which can be the death knell for a small company.
Take pride in your work.
There’s a special satisfaction of playing on a game system I helped design or seeing drilling equipment I worked on in action. But nothing matches the satisfaction of talking to someone whose father’s cancer treatment was improved by a product that I worked on.
It’s even gratifying that the photos of the system never include my camera system. It’s a sign that I accomplished my goal of making my part of the system invisible. That helped prepare me to become a project manager, where our contributions are typically critical, but invisible.